Macrocycles, new modalities to address challenging biological targets
Polyphor’s peptidic and non-peptidic diverse macrocycles designed to mimic key naturally occurring epitopes and proven to be rapidly optimizable.
Successfully tackling complex biological targets
We intend to further leverage the OMPTA class to identify and develop additional product candidates
In collaboration with the University of Zurich, we have established a proprietary macrocycle-based discovery platform consisting of PEMfinder® and MacroFinder®, which was the source of the discovery and development of our product pipeline. Our macrocycles are medium size cyclic molecules (MW-range: 500-2,000 MW) that complement the chemical space between small molecules and large biopharmaceuticals and were designed to address complex and challenging extra- and intracellular biological targets with high unmet medical need. Our macrocycle platform is applicable for many therapeutic areas and different target product profiles. In particular, the platform was successfully used for discovering the novel OMPTA class of antibiotics targeting Gram-negative infections with high unmet medical need.
Polyphor’s product pipeline originates from its proprietary macrocycle technology platform
Macrocycles are medium size, cyclic molecules complementing the chemical space between small molecules and biopharmaceuticals

Protein Epitope Mimetics (PEM) are conformationally constrained cyclopeptides mimicking the biologically most relevant protein surface epitopes such as the β-hairpin and α-helix motifs. PEMfinder® is a highly diverse library derived from sequences of many bioactive peptides including peptide hormones, ligands of G-protein coupled receptors and ion channels, and host defence peptides (Source: A. Luther et al. Curr. Opin. Chem. Biol. 2017, 38, 45–51). By screening the PEMfinder® library and applying PEM technology, promising hits and leads were discovered and optimized, and further developed to our clinicalstage compounds murepavadin, balixafortide and POL6014 as well as the OMPTA platform (Source: A. Luther et al. Bioorg. Med. Chem. 2017, doi: 10.1016/j.bmc.2017.08.006). The MacroFinder® concept is based on non-peptidic, cell-permeable and orally bioavailable macrocycles which can address complex and challenging intracellular targets.
Hot spot binding regions of targets of interest
Macrocycles are ideally suited to address contact surface areas of 400 – 2000 Å2 upon binding.

Polyphor’s macrocycle platform
Our macrocycle library consists of over 50,000 single, untagged, individually purified peptidic and non-peptidic macrocycles readily amenable to all screening formats (binding, enzymatic, cellular, pathway, phenotypical, etc.).
It is a rationally designed, shape-diverse collection of semi-rigid macrocycles. The design is centered around prototypical pharmacophore arrangements mimicking key naturally occurring epitopes typically involved in biological target modulation. Hits from screening the library are typically clustered in families of related compounds already providing a first limited SAR understanding.
This initial advantage is design enabled: We keep exit vector decorations constant across the various scaffolds. We have proven to be able to rapidly optimize macrocycle hit families to leads and beyond with efficient medicinal chemistry driven by structural know-how and highly efficient automated synthesis.
Polyphor offers its macrocycle library to interested parties for screening on their biological targets.
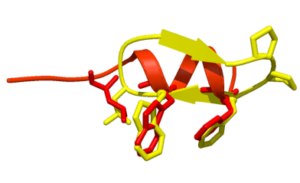 Macrocycles provides diverse functionality and stereochemical complexity in a conformationally pre-organized ring structure;
Macrocycles provides diverse functionality and stereochemical complexity in a conformationally pre-organized ring structure;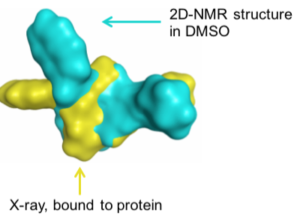 They are semi-rigid compounds. They provide a compromise between structural pre-organization and sufficient flexibility to mould to a target surface and maximize binding (induced fit);
They are semi-rigid compounds. They provide a compromise between structural pre-organization and sufficient flexibility to mould to a target surface and maximize binding (induced fit);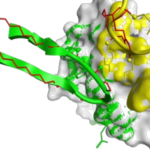 Medium-sized macrocycles can interact with larger protein interfaces typical for protein-protein interactions.
Medium-sized macrocycles can interact with larger protein interfaces typical for protein-protein interactions.